Reviewed and Approved by Tim Lombardo, Sr. Dir. of Food Consulting Services, EAS Consulting Group; Annie Hughes, Director, General Manager FDA Detention, Certified Laboratories
FDA detentions are on the rise. In 2025 alone, the Agency reviewed nearly 50 million line items for products offered for import into the U.S. Many of those shipments were detained for a variety of reasons, leading to an FDA Notice of Action.
If you’ve received a Notice of Action for one of your shipments, it’s vital to act quickly so you can get the shipment released into commerce. In this guide, we walk through each important section and explain how to read an FDA Notice of Action.
How to Read an FDA Notice of Action
The FDA issues a Notice of Action for each entry. It identifies actions for each line item offered for import and is shared with the Filer, Broker, Consignee, and Importer of Record.
The example NOA below includes letters near important fields with corresponding explanations of what each means so you know how to read an FDA Notice of Action.
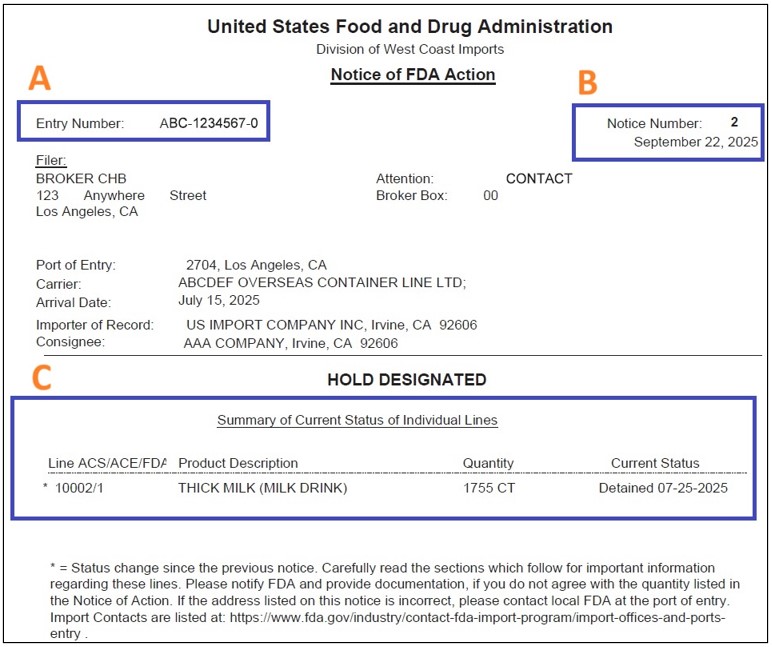
A) Entry Number
Unique number for the entry/case. Use this on all correspondence and uploads.
B) Notice Number
Increments every time the FDA updates the NOA for that entry. The date shown is the issue date of that specific notice.
C) Current Status (per line item)
Shows the status for each product line under “Summary of Current Status of Individual Lines.” See the decoder below for what each status means.

D) Respond-By Date
Your deadline to respond. If you miss it, FDA can refuse the affected line(s). One of your first activities when reviewing a Notice of Action is to note the Respond-By Date. In some situations, you may be able to request an extension. However, it is vital that you act in some capacity before reaching this date to help release your shipment.
E) Reason for Detention / Violation
The legal basis for the action – often referencing an Import Alert – and a short description of the issue.
F) Compliance Officer
Your FDA point of contact who will issue the final decision. Keep communications precise and documented.
G) Terms
Explains your right to provide testimony (e.g., sampling, testing, records) to overcome the violation.
H) ITACS (Import Trade Auxiliary Communication System)
The FDA’s portal to upload documents, check status, and communicate with the agency. Use the Entry Number and Notice Number when submitting. Learn how to set up an ITACS account and use the system here.
“Current Status” on the NOA — What Each Label Means
These status labels appear under “Current Status.” They signal where the FDA is in its review and what happens next.
Pending Review
FDA has the entry and is reviewing paperwork/risk flags. No sampling decision yet. (Listed as a standard status on the NOA.)
Product Collected by FDA
The Agency has sampled the shipment for analysis. Expect a follow-up status once testing or evaluation is complete. (Standard NOA status.)
Detained
The FDA has placed the line under detention. You may not distribute the product into U.S. commerce while detention is in effect; a formal release is required.
Released
The FDA has issued release for that line; product may move into commerce. (Release is the action that ends detention for that line.)
Refused
The FDA has refused admission. The shipment (or line) must be re-exported or destroyed under supervision.
May Proceed
The FDA indicates no objection at this stage; the line may proceed while the Agency finishes entry processing. (Appears as a standard status on the NOA.)
Tips for How to Read an FDA Notice of Action
- Note the dates. As said already, find the Respond-By date and gauge whether you need to request an extension. The earlier you make the request, the better.
- ID the Compliance Officer and initiate communication with that person. Be cordial and respectful – heightened emotions won’t get your shipment out of detention any sooner!
- Match your evidence to the stated reason. Your submission should directly address the violation or Import Alert cited.
- Use ITACS for a clean record. Upload complete, well-named files and reference the Entry and Notice Numbers on every document.
- Contact Certified Laboratories to start the testing and sampling process.
Need Help With a Detained Shipment?
Contact Certified Laboratories right away. Our FDA Import Team has more than 60 years of experience working with companies to get detained shipments released into U.S. commerce.
- Nationwide sampling.
- ISO 17025- and FDA LAAF-accredited testing labs with validated methods.
- Full lab packet submission via ITACS.
- FDA Red List removal services via EAS Consulting Group, a Certified Group company.
