An FDA Import Alert notifies U.S. Food & Drug Administration (FDA) personnel that the agency has sufficient evidence that a product coming into the country may be in violation of FDA regulations. The source of the violation may be the product itself, the manufacturer, shipper, or place of origin.
Import alerts allow for Detention Without Physical Examination (DWPE), which means FDA may detain the shipment without performing a physical analysis. This allows the FDA to efficiently allocate its limited resources to help keep U.S. consumers safe.

What are the Different Types of Import Alerts?
There are four types of Import Alerts with which you should be familiar:
Country- or Area-Wide FDA Import Alert
- The FDA may detain certain products offered for entry from the specified country or area without physical examination.
- For example, Import Alert #12-03 “Detention without Physical Examination of Imported Soft Cheese and Soft Ripened Cheese from France” allows the FDA to detain any cheese from France due to the potential for Listeria.
Manufacturer/Product-Specific FDA Import Alert:
- The FDA may detain certain products from specific manufacturers without physical examination.
- For example, Import Alert #99-08 “Detention without Physical Examination of Processed Human and Animal Foods for Pesticides” allows FDA to detain all processed human and animal foods produced by a manufacturer on the alert’s Red List due to illegal pesticide chemical residues.
Shipper:
- The FDA may detain products from certain shippers without physical examination.
Country/Worldwide Alert:
- The FDA may detain without physical examination certain products from a specific country.
- For example, Import Alert #16-05 “Detention Without Physical Examination of Mahimahi from Ecuador and Taiwan Due to Histamine and Decomposition” allows the FDA to detain all shipments of Mahimahi from Ecuador and Taiwan due to a history of decomposition of shipments originating from those countries. Firms on the Green List of the alert, however, are exempt.
What are the Main Reasons for FDA Import Alerts?
There are two major ways a product is added to the FDA import alert system:
Manufacturing Problems:
- Issues such as unsanitary conditions in a facility.
- These require extensive documentation and inspection to resolve.
- Testing alone cannot resolve these issues; the manufacturing process itself must be corrected.
Product Problems:
- Issues specific to the product itself.
- These can potentially be resolved through testing at the detention stage to prove compliance.
What is Included on an FDA Import Alert?
- Reason for Alert: In-depth explanation of the issues leading to the alert. Common reasons include safety concerns, such as potential contamination or false labeling, and regulatory non-compliance, like missing certifications or violations of standards.
- Guidance for Field Staff: Detailed instructions for FDA field staff regarding the detention and examination of the products in question. This may include specific tests to be conducted or documentation to be reviewed.
- Product Description: Official description of the product.
- Charge: Why the product is subject to Detention Without Physical Examination (DWPE).
- Countries: Information about where the products were manufactured. This can be particularly important if the alert is region-specific.
- FDA Red List: List of firms and their products that are subject to DWPE (aka, the Red List).
- FDA Green List: List of companies that have met the criteria for exclusion from DWPE.
How Do I Find FDA Import Alerts?
The U.S. FDA has a website where you can search for FDA Import Alerts. You can browse by country/area, industry, import alert number, and date issued.
FDA Import Alerts – Red List, Green List & Yellow List
Let’s take a closer look at an Import Alert Red List, Green List, and Yellow List.
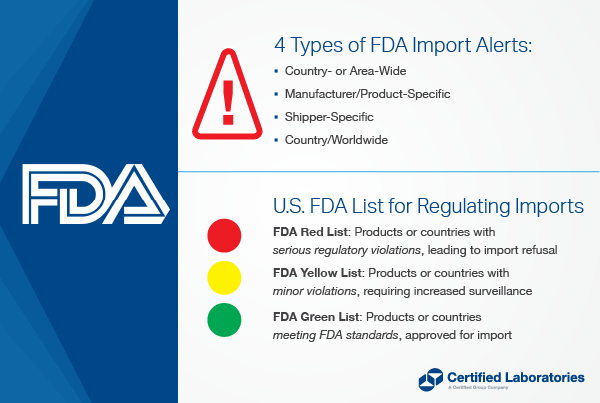
What is the FDA Red List?
An Import Alert Red List includes the firms, products and/or countries that are subject to Detention Without Physical Examination (DWPE).
To illustrate, we’ll look more closely at Import Alert #99-08 “Detention without Physical Examination of Processed Human and Animal Foods for Pesticides” mentioned above. It allows the FDA to detain all processed human and animal foods produced by a manufacturer on the alert’s Red List due to illegal pesticide chemical residues. This means the FDA has reason to believe certain products manufactured by these firms may violate regulations and pose a threat to consumers.
If, for example, ABC Incorporated’s dried raisins are on this alert’s Red List, the FDA will detain any shipment into the U.S. that includes ABC Inc.’s dried raisins.
At that point, the FDA will issue a Notice of Action, which explains why the shipment is detained and provides the opportunity to address the charges and get the shipment released. Company’s may hire a third-party laboratory to perform sampling and testing that demonstrates the shipment is compliant with regulations. Certified Laboratories offers FDA import and DWPE testing.
How Do I Get Removed From an FDA Red List?
To have your products removed from the Red List of an FDA Import Alert, you must…
- Submit a petition to the FDA detailing how the company has identified the source of the problem and is implementing specific, corrective actions that will prevent future violations.
- Typically import five consecutive containers that are free of violations. They must be normal commercial containers; you cannot, for example, divide one commercial container into several smaller shipments to reach the five-shipment threshold faster and be removed from the FDA Red List.
- Those shipments will be sampled and tested by an independent laboratory, and results will be reported back to the FDA.
- If the shipments pass testing, the FDA will clear them and the company can place the products into commerce.
- After sampling and testing, the company can then petition to be removed from the FDA Red List.
We provide detailed instructions for FDA Red List removal in this article.
What Evidence Must I Provide to Petition for FDA Red List Removal?
Depending on the violation, the FDA may require additional evidence to support the petition beyond a third-party lab analysis, such as…
- Manufacturing or processing records GMP/HACCP plans and corrective actions
- FDA establishment inspections
- Proof of registration and/or listing
- Proof of certification by a foreign government or certified body
- Evidence that labeling violations have been corrected
- Evidence that the product is in compliance
Petitioning to be removed from the Red List of an Import Alert is a detailed and complicated process, so it’s best to enlist the help of a regulatory expert who can help, such as EAS Consulting Group, a Certified Group company.
Petitions must include the required information in the proper format or risk denial, which will delay release and drive up your costs.

What is an FDA Green List?
An Import Alert Green List includes the firms, products and/or countries that have met criteria for exemption from Detention Without Physical Examination (DWPE). This is where a company wants to be to streamline the import process and maximize profitability.
To illustrate, let’s look at Import Alert #16-05 “Detention Without Physical Examination of Mahimahi from Ecuador and Taiwan Due to Histamine and Decomposition”. This alert allows the FDA to detain all shipments of Mahimahi from Ecuador and Taiwan due to a history of decomposition of shipments originating from those countries. However, firms on the Green List are exempt.
This means the firm has demonstrated to the FDA it has manufacturing processes and preventative measures in place to ensure shipments will meet regulations and are safe for U.S. consumers.
How Do I Petition to be Added to an FDA Green List?
Like a petition to be removed from a FDA Red List, a petition to be added to an FDA Green List must convince FDA that the firm has addressed the core problem(s) on the Import Alert and has processes in place to prevent the occurrence of violations.
The FDA typically reviews Green List petitions with great scrutiny, so firms should be prepared to address any concerns the FDA may have about compliance and provide additional supporting evidence.
Again, it’s best to hire a regulatory expert to handle your petition given the complexity involved and potential to affect your bottom line. EAS Consulting Group has more former FDA employees than any other company and knows how to get your products released quickly.
What is the FDA Yellow List?
An Import Alert Yellow List includes firms, products and/or countries subject to intensified surveillance; or firms that may have satisfied Good Manufacturing Practices (GMP) issues but where the nature of violations may warrant further field examinations of individual entries and/or additional analyses.
Shipments from firms on the Yellow List are subject to Detention Without Physical Examination (DWPE) and require a private laboratory analysis until the FDA is confident the products are compliant.
Need Help With an FDA Import Alert?
Our FDA Import Team has 60+ years of combined experience helping companies handle FDA Import Alerts and FDA Red List removal. We offer nationwide sampling, validated lab testing, and ITACS submission.
Contact our import experts to get help with your detained shipment.
