Reviewed and Approved by Tim Lombardo, Sr. Dir., Food Consulting Services, EAS Consulting Group; and Annie Hughes, Director, General Manager FDA Detention, Certified Group
1-Minute Summary
- FDA Red List removal requires proving the issue that caused detention has been fixed and prevented from recurring.
- Depending on the type of import alert, many petitions require evidence of at least five consecutive, commercial-size, non-violative shipments tested by a third-party lab experienced in FDA testing.
- Strong documentation, including root cause analysis, CAPA plans, and updated Food Safety/HACCP plan, and inspection records, is essential for petition approval.
- Submitting small test shipments or skipping documentation are common mistakes that delay or derail your petition.
FDA Red List Removal Requires a Step-by-Step Plan
No one in the importing industry wants to deal with the delays, costs, and strained customer relationships that come with FDA detention. We’ve covered the basics of FDA Import Alerts, including how to read an Import Alert, in previous articles. Today, let’s focus on the concrete steps you should take to resolve an Import Alert and achieve FDA Red List removal.
Step 1: Know What the FDA Needs to See
To get off a Red List, you’ll need to show the FDA two things:
- You’ve resolved the issue that caused the alert (adulteration, mislabeling, unsanitary conditions, etc.).
- You’ve taken steps to prevent it from happening again.
This usually requires submitting a formal petition with detailed supporting documentation. Depending on the violation, that documentation might include:
- Results from a third-party lab that specializes in FDA testing and that shows no contamination (e.g., pathogens, pesticides, heavy metals).
- Manufacturing records, food safety plans, and corrective actions.
- Proof of clean shipping history – often five consecutive, commercial-size shipments that pass FDA review.
- Inspection reports from the FDA or foreign authorities showing your facility is compliant.
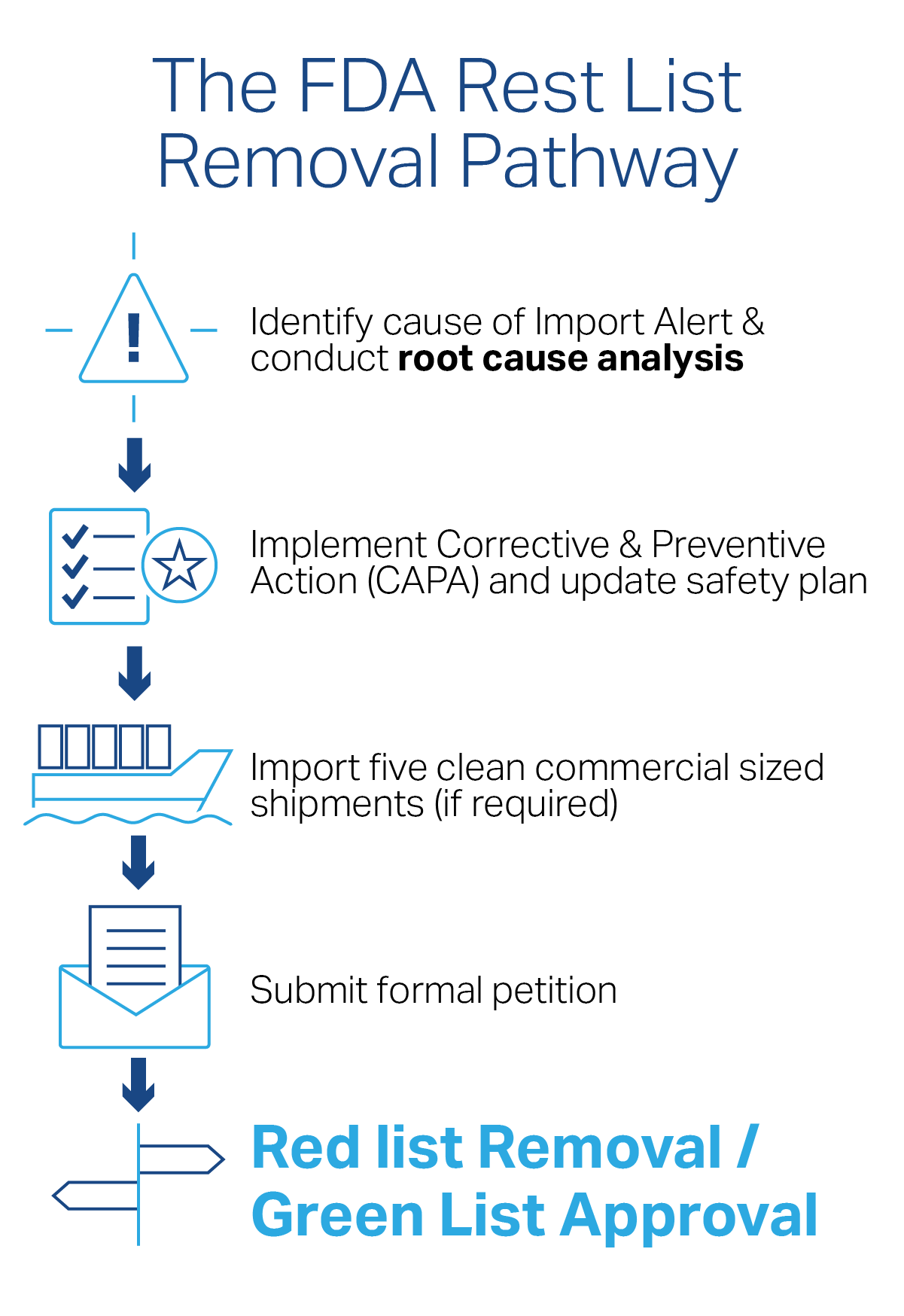
Step 2: Execute a Corrective and Preventive Action Plan
The FDA doesn’t just want to see a quick fix. They want to know the issue has been investigated, corrected, and prevented.
Start with a root cause analysis. Ask: What caused the violation? Was it poor sanitation? Faulty documentation? Ingredient contamination?
Then develop two plans:
- Corrective Action Plan (CAP): How did you fix the issue?
- Preventive Action Plan (PAP): What changes will stop it from happening again?
If you’re importing food products, update your Food Safety Management Plan to reflect both. This is not optional. Without documented procedures and monitoring steps, your petition is likely to be rejected.
Step 3: Submit Five Clean Shipments (Typically)
To support your petition, you’ll often need to import five consecutive commercial-size shipments that:
- Have been detained by FDA under the Import Alert.
- Have tested clean or with non-violative results by a third-party lab using FDA-accepted methods.
- Are spaced out in a “routine” shipping pattern (not five shipments sent in one week, for example).
- Are of commercial value (at least $2,500 per shipment to qualify as formal entries).
These shipments prove to the FDA that your product is safe and that your fixes are working.
Important: If even one shipment in the sequence violates FDA standards, you must start the five-shipment process all over again. Additionally, if a shipment is inadvertently not detained as part of the Import Alert, it will not count as one of the required consecutive shipments. Further, in some cases, more than five consecutive shipments may be required. In some cases, the five non-violative-shipments requirement does not apply, such as if the initial reason for detention was due to a labeling problem.
Step 4: Send Your Petition for FDA Red List Removal
Once you’ve compiled your documentation and clean shipment evidence, it’s time to submit your petition.
- Email your petition to the address listed on the specific Import Alert.
- Include all required documentation in the format the FDA expects.
- Avoid sending repeat status update requests – these do not speed things up and may even slow down your review.
Your product remains on the Red List throughout the FDA’s review. If your petition is accepted, you’ll receive a letter confirming your removal or your addition to the Green List. If it’s denied, the FDA will explain what you missed so you can revise and resubmit. Additionally, if any shipment is in violation during the FDA review, the petition will be denied, and the process is required to begin again.
Avoid These Common Mistakes When Petitioning for Red List Removal
Here are five missteps that often lead to denial or delays:
- Incomplete Corrective Action Plans – Skipping root cause analysis or failing to document changes.
- Non-commercial shipments – Sending sample-size or test-only shipments instead of full loads.
- Shipments not detained by FDA – They must be detained to count.
- Shipments too close together – Send them as part of your regular business schedule.
- Missing documentation – Even one missing COA or inspection report can delay your review.
Example: Import Alert 99-30 – Milk Products from China
Let’s illustrate the FDA Red List removal process with an example of importing milk-based products from China. Because of past incidents involving melamine contamination, the FDA issued Import Alert 99-30, placing all such products under DWPE.
To get your company on the Green List, you’ll need:
- Five consecutive commercial-size shipments that test free of melamine and cyanuric acid using FDA-accepted methods.
- Documentation from a trusted third party showing your facility has systems in place to prevent melamine contamination.
- Inspection reports proving your manufacturing conditions are safe.
- Verification that your company complies with Chinese export regulations, including facility registration.
The FDA may require an onsite inspection or a review of your third-party auditor’s work before approving your petition.
Example: Import Alert 99-43 – Ready-to-Eat Foods and Unsanitary Conditions
Here’s another example. Imagine your facility produces ready-to-eat (RTE) foods like sandwiches or frozen meals. Due to findings of unsanitary conditions, like improper sanitation or contamination, your company ends up on Import Alert 99-43.
To be removed from the Red List, the FDA expects:
- A root cause analysis that clearly identifies how contamination occurred.
- Documentation showing corrective actions (e.g., equipment repair, revised cleaning SOPs).
- Evidence that preventive measures are now in place, such as environmental monitoring and employee training.
- A written Food Safety Plan that includes hazard analysis, preventive controls, corrective actions, verification procedures, and a recall plan.
- Five clean, commercial-size shipments that test negative for microbial hazards like Listeria, Salmonella, or E. coli.
- Optionally, the FDA may conduct a follow-up inspection to verify your information.
This level of detail proves to the FDA that the problem is not only fixed but unlikely to recur.
FDA Red List Removal Can Be Done – But Have a Plan!
Resolving an Import Alert and achieving FDA Red List removal can sound like a daunting task. But the payoff is huge, especially if you land on the Green List.
If you’re not sure where to start, Certified Group companies can help. Certified Laboratories provides nationwide sampling and lab testing, including ITACS submission. EAS Consulting Group can help with Red List removal, label reviews, and other regulatory consulting needs.
Contact us now if you need help with a detained shipment or Red List removal services.
