In this post, we will cover a few important points about U.S. customs, the U.S. Food and Drug Administration (FDA) and some modern-day challenges faced by food importers and exporters. We’ll also provide a general understanding of the entire food import process.

Here’s what we’ll cover:
- Brokers & Automated Commercial Environment (ACE)
- Diagram of Proper ACE Process
- Common FDA Issues Importers Face & How to Understand Them
- Who is the Importer?
- What is an FDA Hold, Detention, and Import Alert?
- How to Read an FDA Product Code
- What Products Entering the U.S. Does FDA Regulate?
- Entry Review Process Specifics
- When are FDA-Regulated Products Refused?
- What is Detention Without Physical Examination (DWPE)?
- What if FDA Refuses Your Product?
- Import Refusal Overview
- Different Types of Import Alerts
- What is a Red List & Green List?
- The Role of Third-Party Laboratory Testing
- What to Look for When Hiring a Third-Party Laboratory
- Final Submission Packet to FDA ITACS
- What is the Foreign Supplier Verification Program (FSVP)?
- Correcting Foreign Supplier Verification Program (FSVP) Violations
- When to Seek Help from Consultants
- FDA Import FAQ
Brokers & Automated Commercial Environment (ACE)
Most importers use a U.S. customs broker to facilitate the clearance of cargo coming into the country. Brokers transmit all data requested by the importers or from the suppliers as the cargo is prepared to enter the country. If you’re part of the industry, understand that ACE is the “Automated Commercial Environment” that Customs & Border Patrol (CBP), the agency in control of all transmissions for a product coming into the country, uses to transmit all communications.
Prior to transmitting any information, it is the broker’s responsibility to ensure that all documents received match all the documents presented, meaning that all the documents you submit as importers or as suppliers are properly prepared and make sense.
The broker transmits entries to FDA and all other partner government agencies through ACE simultaneously with the transmission from the CBP cargo clearance. This means that along with all the data that’s requested for CBP, additional information or data elements are required for FDA clearance of your product.
Additionally, brokers work as facilitators of information between the government agencies and the importers, meaning they are the “megaphone” for you as an importer; they are the individuals who transmit all the initial data that allow for the government agencies to see the information that you are presenting through your bill of lading, airway bill, invoices, packing list and any additional documents that are required for the clearance of FDA product.
Diagram of Proper ACE Process
This diagram helps provide a general understanding of how the import system works:
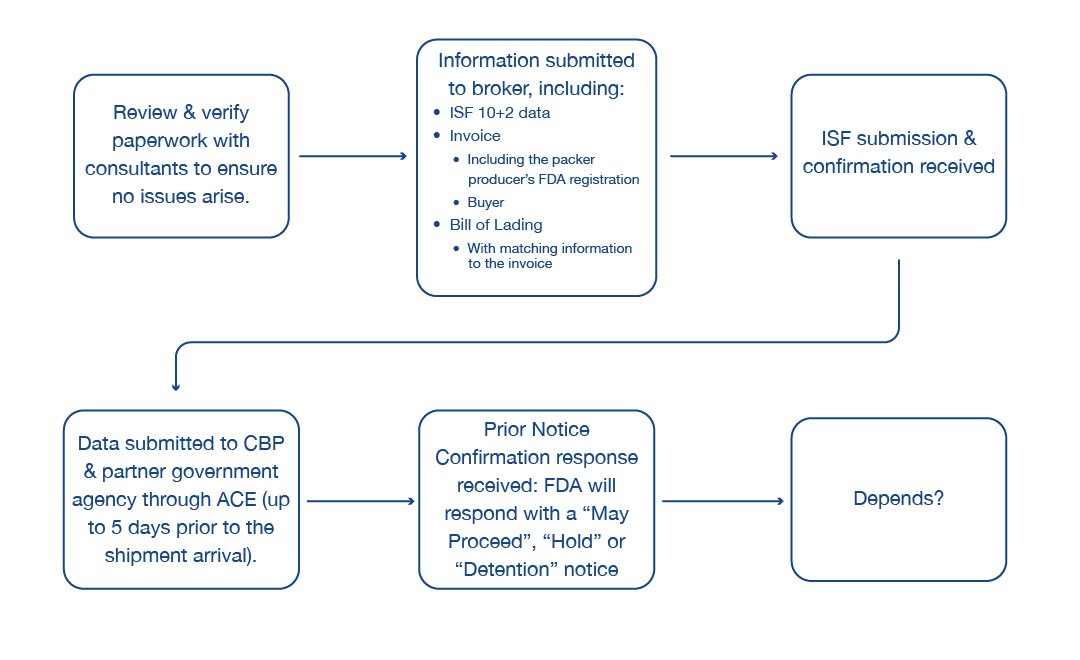
The most important piece is the review and verification of paperwork with consultants to ensure no issues arise. In many cases, verification that all the documents match before any data is sent to your broker or before any data is transmitted through the ACE system is the most important step. It’s also important to determine if something is on an Import Alert, which we’ll cover later.
Next, the information that is collected is verified and transmitted by the broker for an Import Securities Filing (ISF) 10+2. This includes the initial 10 data elements that must be submitted 24 hours prior to the cargo’s departure at origin. In addition, the information needed to be able to present customs clearance includes the invoice, which includes the packer producer’s FDA registration; the information that identifies the buyer and seller; the manufacturer’s packing list; and your bill of lading, all of which should match the information associated to the invoice.
Once the ISF 10+2 is submitted through the system, a confirmation is received that CBP has received that initial data. The cargo then departs and, throughout the entire course of its transportation from the location of origin to its destination in the United States, the data-collection portion of the actual exercise of any clearance takes place.
During that time, the broker, or importers themselves, collect all the data and ensure that it is properly prepared to be submitted through the ACE system to CBP. Once it enters CPB’s system through the ACE system, CPB then transmits the additional information to its partner government agencies that are required for that clearance.
Typically, the data can be transmitted up to 30 days prior to the cargo’s arrival. Five days prior to its scheduled arrival date, FDA will then give a notification or answer. Once the information is received and FDA confirms receipt, the broker receives a Prior Notice Confirmation response through the ACE system. In that time, FDA will respond with either a May Proceed, Hold or possibly a Detention notice.
The final step depends on several factors, most notably if you receive a Detention notice. We’ll cover potential next steps below.
Common FDA Issues Food Importers Face & How to Understand Them
- Who is the importer?
- What is an FDA Hold, Detention, and Import Alert?
- Why is an FDA product code important?
Who is the Importer?
People often think the importer is whoever is declared as the importer of record for CBP. However, that’s not the exact case. There can be many situations where the CBP importer of record is not the same as the FDA importer of record.
CBP Importer of Record
- Individual responsible for the customs clearance and liquidated damages if fines or redelivery notices are issued. This means the redelivery is issued to the CBP importer of record, which will be found liable if the shipment is not properly handled.
FDA Importer of Record
- Individual responsible for the shipment for FDA purposes.
- Required to answer for any recalls that FDA may issue.
The FDA importer of record is responsible for anything involving the FDA. However, when a redelivery notice is issued, it’s issued through CBP. Therefore, the CBP importer of record will be responsible for the redelivery of the product that may be violative by the FDA importer of record. In most cases, they are the same individual, but it’s vital to understand that they may be different.

What is an FDA Hold, Detention, and Import Alert?
Let’s look at an FDA Hold first. An FDA Hold can be issued because of an Import Alert, a random occurrence by FDA’s PREDICT system or for any of a variety of reasons associated with additional shipments that may be coming in by different importers. Therefore, an FDA Hold is the first step into what you do next (unless you receive a May Proceed notice).
If FDA requests samples, it’s considered a random sampling. It does not mean that there will immediately be a private lab analysis. It means FDA is sampling for its own purposes based on its own data, and it’s a normal piece of the import puzzle. However, when a shipment is detained due to an Import Alert, you must consider other factors discussed below to be removed from the Import Alert.
In many cases, the initial step for the FDA Hold is a request for documentation. That documentation will require you to present the same documents submitted to CBP through the electronic transmission to be backed up with the actual paperwork that comes with that shipment. In that information, FDA is looking to match the information that was initially submitted with what they have in their own database.
This URL allows you to view all the different Import Alerts by country, industry, the number assigned to each alert and publication: https://www.fda.gov/industry/actions-enforcement/import-alerts#list
This is a direct FDA system that importers and exporters should use to verify whether your suppliers are in good standing with FDA. It’s one of the first steps on the diagram above that helps you review information to ensure you don’t run into problems once the cargo has already been purchased and you’ve already paid a deposit on your cargo. You don’t want to be stuck dealing with this issue as the cargo is coming into the United States.
Next, let’s look at an FDA Import Alert. Here, you can see an example of an Import Alert, specifically Import Alert 16-05:
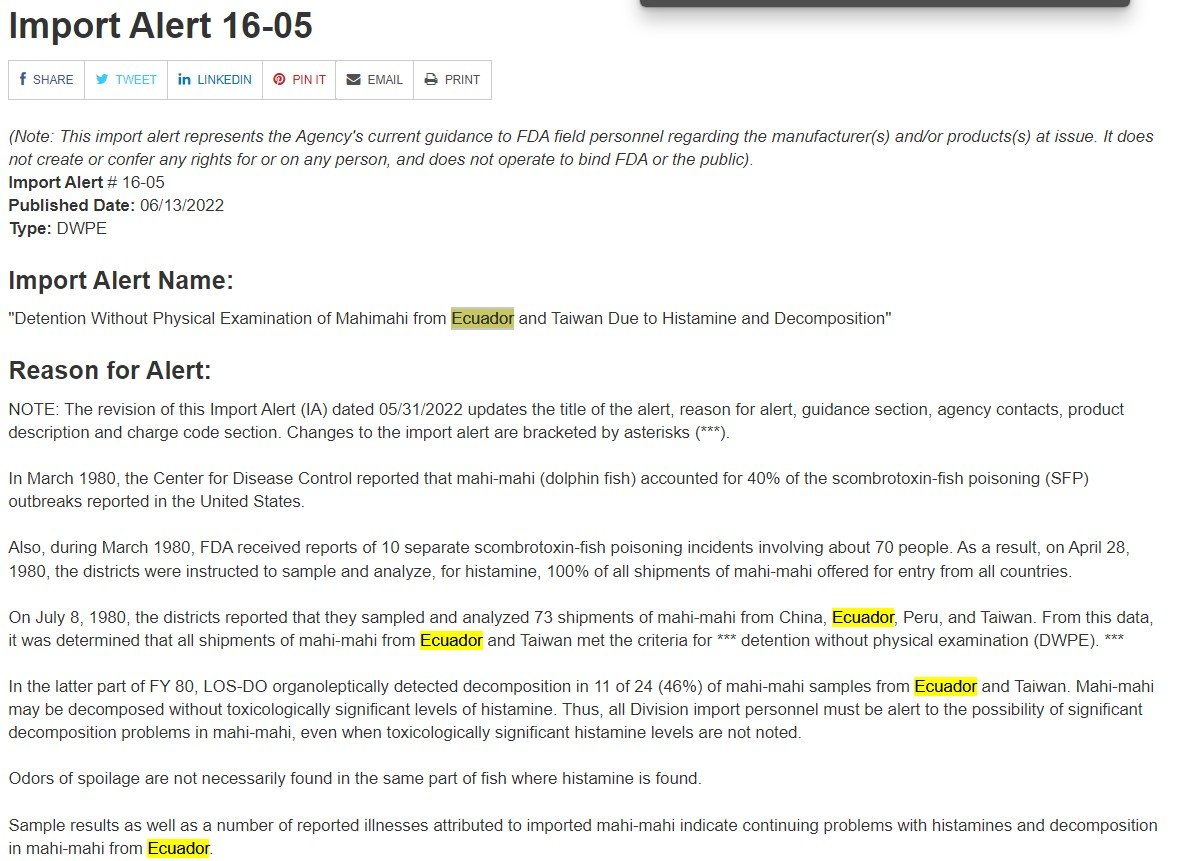
This Import Alert deals with Mahimahi that may have histamines and decomposition by findings of FDA. First, you can read the reason for the Import Alert. You can also see the different countries that are of concern to FDA followed by the guidance of FDA for these different products:

Next, you can see that this alert is countrywide, meaning any product coming from Ecuador or Taiwan immediately falls under this Import Alert. Therefore, when FDA receives any information that lists Ecuador or Taiwan as country of origin or country of production, FDA will detain this product because it’s coming from one of these countries:
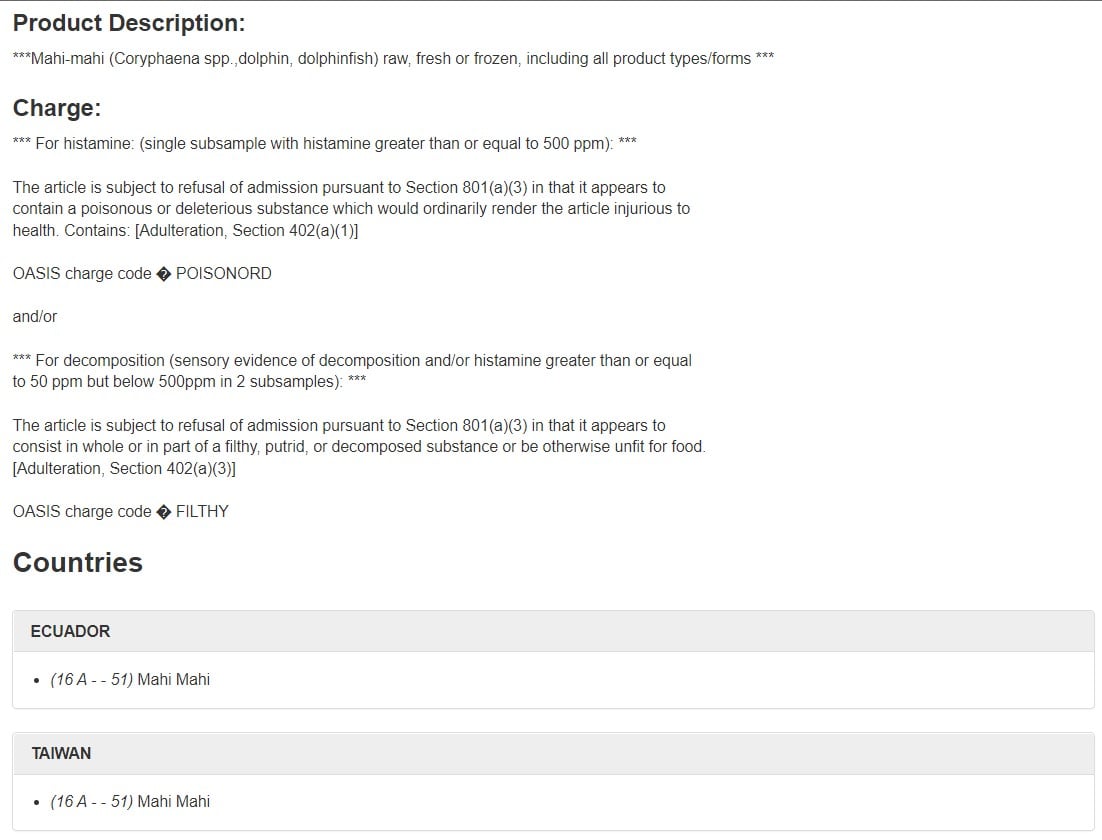
Many Import Alerts include a Red List, which we’ll cover later. They often also include a Green List, which lists the firms and their products that have met the criteria for exclusion from Detention Without Physical Examination (DWPE), also discussed later.
How to Read an FDA Product Code
Let’s look at how to read an FDA Product Code using the example below:

In this example, the Product Code is 16 A – – 51. It is one of the ways that FDA subcategorizes the product that is being imported, along with the tariff classification submitted through CBP. Here’s a breakdown of what each character means:
Industry Code, Class, Subclass, Process Indicator Code (PIC), Product (Group)
In this example…
16 = Fishery/Seafood Product
A = The product is fish
G = The packing
T = Its status
51 = The product itself
The Product Code is one of the methods FDA uses to flag products for review of Import Alerts by FDA officers.
What Products Entering the U.S. Does FDA Regulate?
The FDA regulates a wide range of products, including foods (except for aspects of some meat, poultry and egg products, which are regulated by the U.S. Department of Agriculture); human and veterinary drugs; vaccines and other biological products; medical devices intended for human use; radiation-emitting electronic products; cosmetics; dietary supplements and tobacco products.
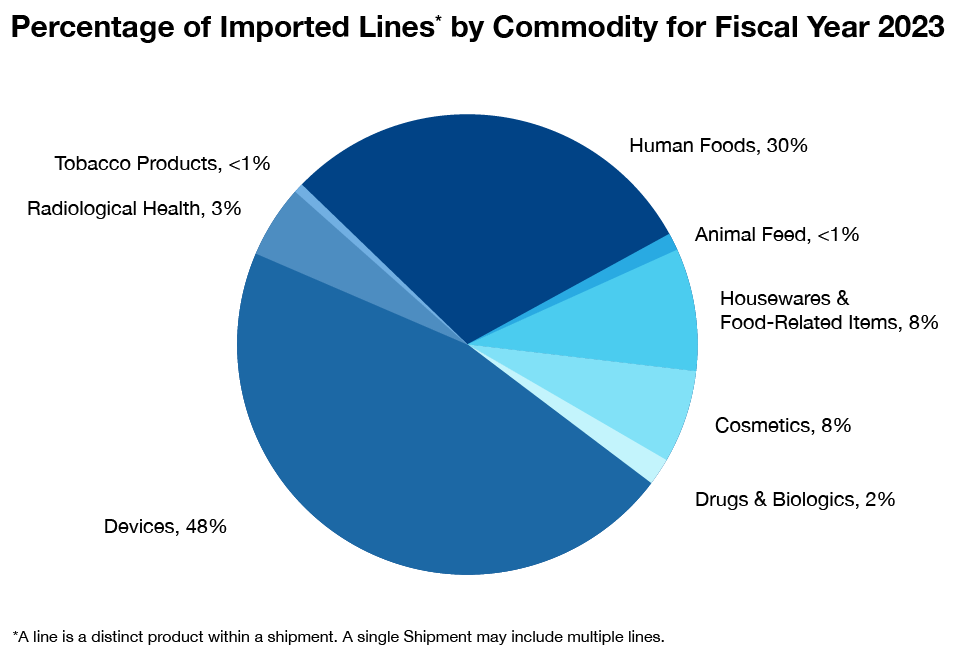
Imports Increase 5-10% Each Year
We have found over the last 10 years or so that the U.S. is importing more products into the country every year for various reasons, including cost of production in the U.S. and lack of materials required to support consumption.
It’s an ever-growing challenge for FDA due to the agency’s limited resources, meaning FDA must find efficient and effective ways to monitor imports, especially for food safety.
Entry Review Process Specifics
If FDA issues a Hold or Detention on a product, the product must be held and not brought into commerce prior to authorization by FDA for release. It could be held in a storage warehouse or a freezer warehouse; it must be set aside and held until such time as FDA allows it to be released.
This can be costly to a lot of importers, so it’s best to do your due diligence in advance. Know what you can expect when the product comes into the United States and if FDA is going to be monitoring it. There are a lot of products that are not what’s called “low-hanging fruit”; they’re typically produced in sanitary conditions; the factories they’re produced in have good HAACP plans, good production plans and good safety programs; and those products run through the United States without FDA looking at them.
It doesn’t always work this smoothly, however. FDA has inspectors around the world who inspect factories and determine whether a factory or a country of origin is having any issues. If so, FDA will look more closely at shipments into the United States from those places.
When are FDA-Regulated Products Refused?
FDA-regulated products are refused entry if they appear to be or have been found to be:
- Adulterated, meaning the product is contaminated, is not safe, unapproved, or does not otherwise meet applicable standards
- Misbranded, meaning the labels contain false or misleading information; or the product is not registered and listed, if required; or the nutritional panel does not meet U.S. standards
- Forbidden or restricted for sale
“Adulterated” means the product is contaminated, unsafe, unapproved and doesn’t otherwise meet FDA standards for good manufacturing practices (GMP). Labeling issues have become a big problem. It’s difficult to stay on top of the changes that are going on with labeling. People want to put things on their labels that aren’t allowed, or they’re not formatted properly. FDA will stop the shipment and require you to relabel the product, or they will refuse it. If FDA refuses your product, you have 90 days either to destroy it, show that you brought the product into compliance, or export it. It is required that FDA witness the destruction of the product.
What is Detention Without Physical Examination (DWPE)?
If a product is detained without physical examination, FDA has demonstrated via testing that the product has been shown to be violative. For example, testing could have revealed salmonella contamination or antibiotic residue. If that’s the case, the manufacturer will be put on the Red List, and that product will automatically be detained each time it’s brought into the country. You will not be able to distribute the product into the U.S. if it’s detained prior to having a third-party laboratory perform an analysis to prove the product is within compliance (a.k.a. “provide testimony”).
The Purpose of DWPE
FDA has limited resources, and thousands of containers are imported into the United States every year. The agency simply doesn’t have the resources to check every shipment, so they use their PREDICT system, which allows them to look at the “low-hanging fruit” to identify the products most likely to have issues. This allows FDA to direct its resources accordingly.
What if FDA Refuses Your Product?
The Federal Food Safety Modernization Act (FSMA) was enacted to ensure the safety of the U.S. food supply by, in part, shifting focus from Federal regulators responding to contamination to preventative measures. This gives FDA a mandate to enact additional preventive controls to ensure food safety. FSMA has four key goals:
Food offered for import meets U.S. food-safety requirements
- This optimizes FDA foreign-facility inspectors and resources while holding foreign suppliers to a U.S. safety standard
FDA border surveillance prevents entry of unsafe foods
- FDA personnel undertake field operations that focus on import screening and the import-review process
Rapid and effective response to unsafe imported food
- This maximizes the effectiveness of FDA involvement in the food-import process, which enhances the effectiveness of food-safety recalls
Efficient and effective food-import program
- Dedicated to resource allocation and to ensure the effectiveness of importing through the performance of continuous improvement
Food Import Refusal Overview
An import refusal always starts with an FDA Notice of Action. It’s normally sent to the filer, importer, owner and/or consignee. They normally specify the nature of the violation, as shown in the example below:
Refusal of admission pursuant to Section 801(a)(3) in that it appears to bear or contain an unsafe food additive (INS 900a) within the meaning of section 409. Adulteration, Section 402(a)(2)(c)(i), Section 801(a)(3) of the FD&C Act in that it appears to be misbranded (“COLOURING COCHINEAL CARMINE”) within the meaning of Section 403(i)(2) of the FD&C Act.
In this example, the nature of the violation is that a product appears to contain the food additive “cochineal carmine”.
When you receive a Notice of Action, you are entitled to various options, including…
- An informal hearing to provide testimony regarding the admissibility of the product
- Submission of evidence that the product is compliant
- Evidence will include either a label review or laboratory analysis, depending on the specifics of the refusal
Failure to submit evidence or a plan to bring the product into compliance results in only two options:
- Destroy the product under FDA and CBP supervision
- Incurs added cost since FDA or CBP will send personnel to supervise
- Export the product under FDA and CBP supervision
If you receive a Notice of Action, it’s important to review it and respond immediately, as we explain in this post.
Exporting the product carries several potential difficulties, which is why it’s recommended that you contact an expert, such as EAS Consulting Group (a Certified Group company), for assistance.
You may be required to contact the country that is going to accept the shipment. In one case, a product detained for Listeria was refused entry into the United States due to the country’s zero-tolerance policy. But the European Union has different tolerance levels. The issue that held up the product was that the importing country wanted more paperwork to assure the safety of that product since they don’t want a product that was refused by FDA; once they see a refusal, they want more information, and a consultant can help with that.
A refusal is normally a final decision. FDA may rescind a refusal if they find that they have made an error, but that’s very seldom. Unless the refusal was issued in error, consideration is not given to a request to rescind a refusal. EAS Consulting Group, a Certified Group company, can help you with this.
FDA has no authority to grant extensions. Normally, you must deal with the local CBP office to extend the 90-day period. However, CBP extends very few cases due to their limited resources.

Different Types of Import Alerts
Let’s take a closer look at import alerts.
Country- or area-wide alert (products offered for entry from the specified country or area). Example: 12-03 Listeria for cheese from France. Any cheese from France will automatically be detained. If a company in France has proven that it has good manufacturing practices and it has brought in a minimum of five containers shown not to be in violation, the company will be put on the Green List and can ship the product into the United States without delay.
If a manufacturer has been found violative, it must import five containers – they must be normal containers; you cannot ship, for example, 100 pounds of product to try to be removed from a Red List. You must go through five typical shipments. Those shipments will be tested by an independent laboratory, and results will be reported back to FDA. If the product passes the test, FDA will clear those five shipments. Afterward, the company can petition the FDA for Red List removal.
Manufacturer/product-specific alert (certain products from specific manufacturers). Example: 99-08 Pesticides.
Shipper (certain products from shippers).
Country/worldwide alert (certain products from all countries outside the U.S.). Example: 16-05 Mahimahi from any country for Histamine and decomposition.

What is a Red List & Green List?
Red List: Firms, products and/or countries are subject to Detention Without Physical Examination (DWPE) under an Import Alert. To have your products removed from the Red List of an Import Alert, you must submit a petition to FDA detailing how the company has identified the source of the problem and is implementing specific, corrective actions that will prevent future violations. Check out this post for more information about being removed from a Red List.
Green List: Firms, products and/or countries that have met criteria for exemption from Detention Without Physical Examination (DWPE) under an Import Alert.
The Role of Third-Party Laboratory Testing
FDA is obligated to try to keep Americans safe by ensuring the foods coming into the United States are safe. The agency uses Import Alerts to use their resources effectively to minimize the number of shipments that must be detained. Only 2-3% of containers are examined due to FDA’s limited resources.
FDA believes that, given agency resource limitations and increasing import obligations, they are obligated to rely to a significant degree on data generated by private laboratories to make compliance decisions.
What to Look for When Hiring a Third-Party Laboratory
If you’re going to hire a third-party laboratory to test your detained products, their submission to FDA must include certain documents in the submission package. Certified Laboratories (a Certified Group company) uses the following process to gather the required documents:
FDA Inquiry
- Request Entry Documents
- The FDA Notice of Action (which you will receive if FDA detains your shipment)
- Packing List
- Commercial Invoice
- Warehouse Tally (shows product location and any identifiers that explain where the product is inside the warehouse)
Review Documents
- Import Alert on the Notice of Action
- Product location
- How is the product packed?
- Determine number of lines/lots/product codes
Order Confirmation w/Quote
- Certified Group sends an order confirmation, upon approval sampling is scheduled.
Write Collection Report
- We compile your report based on the packing list and the commercial invoice. FDA has criteria for the number of portions of each line we must collect. Depending on the Import Alert, it can be expensive. To reduce costs, it’s best to minimize the number of product lines and the number of product codes included in each shipment because FDA has been pushing for sampling and testing of each production lot.
- Determine sampling plan based on the testing
It’s Best Practice to Hire a Firm to Handle the Sample Collection
Many people want to complete the collection process and report on their own. While allowed, we don’t recommend it due to the complexity involved. Certified Laboratories (a Certified Group company) has certified collection personnel who know the process.
One issue currently confronting labs is the difficulty in accessing warehouses since the COVID-19 pandemic due to shortages of warehouse storage and personnel. It’s important to keep that in mind when importing into the United States.
Final Submission Packet to FDA ITACS
In addition to the appropriate forms mentioned above, Certified Laboratories includes the following items in our final submission to FDA:
- Photo report of the sampling process
- Analysts’ training CVs
- List of each piece of equipment used in our analysis, including calibration
A submission can be up to 400 pages. Once the analysis is done, we send an “importer’s statement”, which states that you have not had the product tested by multiple laboratories. If you had, you must disclose that on the form. Some unqualified laboratories try to complete this process, which results in FDA rejecting the submission. In that case, you must find a second laboratory to complete the process, and you have to disclose that you used a laboratory the first time, but the submission was rejected.
Currently, the importer owns any analysis that’s been done by a laboratory, and it doesn’t have to be reported to FDA. That may change in the future; regulators are working on new regulations. But, until you give us authorization, we do not report to FDA. Once you give us authorization, we upload the package into the FDA’s ITACS online portal for review by their scientists to ensure that the sampling and our analysis was done in accordance with FDA protocols.
What is the Foreign Supplier Verification Program (FSVP)?
FDA’s Foreign Suppler Verification Program (FSVP) has different priorities, including…
Compliance History: History of non-compliance [recalls, laboratory class 4, microbiological findings, Official Action Indicated (OAI) & foodborne-illness outbreak].
High-Risk Foods: Foods that may pose a health risk (ready-to-eat, undeclared food allergens and foods requiring process controls, like pasteurization & refrigeration).
Other Supply Chain Factors:
- Number of different foods imported by the importer
- Number of suppliers from which you’re importing food
- Volume and value of the lines of food that are imported
The FSVP requires the importer to perform certain risk-based activities to verify that human and/or animal food you’re importing has been produced in a manner that meets applicable U.S. food-safety standards.
Normally, after FDA completes its inspection, the import company is placed on notice for lack of FSVP for a number of imported food products. The warning letter will include specific language that indicates a failure to develop, maintain and follow FSVP.
FDA will be very specific to what they found during the inspection. They may not limit themselves to the products they reviewed on that occasion but may include all the products FDA may consider under the risk-based activities. It’s important that you examine the particulars of the warning letter, which is something a consultant, like EAS Consulting Group, can help you navigate.
Correcting Foreign Supplier Verification Program (FSVP) Violations
You have a limited amount of time to respond to an FSVP Warning Letter, so it’s important to compile and complete the right documents. It’s your opportunity to demonstrate you have made the required corrections and are following FSVP regulations. Documentation includes…
- Evaluation of your corrections (e.g. documentation of changes)
- Copy of your FSVP & records that demonstrate implementation
- Any additional information relevant to demonstrating compliance with FSVP regulations
You must respond in writing to the FSVP Warning Letter within 15 working days of receipt. If you fail to address FDA, they may take further action. In the case of imported food products, FDA may detain and refuse violative entries, or place imported food products on Import Alerts to inform FDA field staff that they may detain (i.e. initiate a refusal of admission) future shipments of a food without physical examination. One example includes Import Alert 99-41 “DWPE Human & Animal Foods Imported from Foreign Suppliers by Importers Who Are Not In Compliance with the Requirements of the Foreign Supplier Verification Program (FSVP) Regulation”.
When to Seek Help from Consultants
Your level of experience as an importer should influence whether you navigate the import process yourself or seek the help of a consultant. If you haven’t faced some of the issues outlined above, consider reaching out to a consultant to help avoid common pitfalls. Consultants can access the required information quickly and help alleviate many of these issues so you can focus on getting the product into the country at the right cost.
What are some signs that you should reach out to a consultant? If you field questions from your importer or broker that you can’t answer:
- What’s the FDA registration number for this product?
- In what form is it coming into the country?
- Do you know if it’s on a Green List or Red List?
- Does the country have any sort of a hold?
The inability to answer these questions is a key indicator that you should consult experts to help with the process.

FDA Import FAQ
Who creates the number of lines on an entry?
The lines are created based on the data transmitted through the ACE system. That information is based on the paperwork submitted, which should be segregated and disseminated based on product similarity. For example, because Mahimahi is packed in different sizes, you would have to add each additional size to the one harmonized tariff schedule code folder you submit to customs. You would add each line with the weights, values and supplier associated to each. Work with a customs broker to ensure that the product lines are properly documented.
How can a manufacturer/exporter check if they’re on the Green List or Red List?
You can view the FDA Import Alert List and look at the country of origin. Using “CTRL+F”, type in the manufacturer’s name and, if they are on either the Red List or Green List, you can see their status. It can be a little confusing sometimes because some Import Alerts only have a Green List or only a Red List. If your name is not on the Green List, you will likely be detained, and vice versa for the Red List.
Which documents for each shipment must be originals and which can be copies? Should they be attached to the shipment, such as COOs or CITES documents that require signatures?
Normally, the documents are the same as those you provide to your import broker, and they are considered originals once the import broker receives them. If you receive them as an attachment from your supplier or foreign manufacturer, you can supply them in that format. FDA checks that the documentation matches the original entry that the broker provided. FDA will accept a PDF copy from the foreign manufacturer – it doesn’t need to be certified by any other entity; it just has to match.
One thing to consider, however, is that CITES deals with products that, while regulated by FDA, are also regulated by U.S. Fish & Wildlife. CITES documents in particular must be the originals and submitted to an office where there is an actual service port. Those documents can’t be electronic and must be submitted because they’re certified by different government agencies, and you’re required to present them to U.S. Fish & Wildlife.
You may also need original documents if your clearance is done with original bills of lading versus a Telex release associated with the importation, meaning the cargo travels with a document that is considered an original bill of lading. It’s like owning a title to a product, and whoever has the physical document has title to that product and has the right to liberate the cargo from the port by presenting the document. It’s similar to a certified receipt, like how a car title is associated to each vehicle.
How do I handle importation of products for which UPS or Fed-Ex require me to sign? Do I need an additional customs broker beyond the services provided by UPS and Fed-Ex?
Depending on the size of the shipment, UPS and Fed-Ex asks you to sign a document that makes you the importer of record. This allows them to present clearance on your behalf if they are the broker or if you use your own customs broker. They ask you to sign the document so they can identify the responsible party for import purposes and customs & duties while identifying the importer of record and responsible party for FDA.
If you have already cleared cargo and have a good understanding of the requirements for your product, you can use the UPS or Fed-Ex service. If you don’t have an understanding or if you’re in a time-sensitive situation importing fresh product, you may want to use a broker that’s specialized in those types of products to avoid the slowdowns associated with dealing with a large company like UPS or Fed-Ex.
What services does Certified Group provide to people importing products into the U.S. from foreign countries?
FDA allows you to send samples to a lab for testing only and not for entrance into commerce prior to shipping the product. While that will not necessarily allow you to import the product since our testing would only be for your purposes and won’t affect the FDA’s activities, it does offer you peace of mind.
It’s important to understand that, when dealing with research and development, one of the intended uses that can be submitted by the broker is that the product is being imported for another purpose other than commerce in the United States. If your broker does not submit that information to FDA, then FDA doesn’t know the difference between an R&D product and a commercial shipment. Contact Certified Laboratories to find out how we can help.
What questions should I ask to find the right customs broker?
- What PGAs (partner government agencies) are involved with the products you’re importing?
- Make sure to say “PGA”; the broker should have that information and, if not, they don’t know what a partner government agency is, they’re not dealing with anybody other than CBP and they may not have the expertise needed.
- What original documents are necessary for certain shipments?
- How do you transmit information?
- Almost all transactions for CBP and FDA are electronic; very few customs brokers use physical paper anymore, so if they can do business electronically and they answered your other questions, then consider whether you want to do business with them. If they still use paper forms, know that it will increase the time needed to transmit your information to the FDA office. In addition, the FDA reviewer will need to review those papers individually, which takes time.
- Who’s your software provider?
Who is required to have FSVP?
It is the importer – the facility that receives the product, not the customs broker. The customs broker is only a conduit to submit the documentation for FDA clearance, but FSVP is the responsibility of the importer itself. EAS Consulting Group offers a number of services related to FSVP, so visit www.easconsultinggroup.com for information.
What do I look for when choosing an FDA US Agent?
Enlisting an FDA US agent is a basic requirement for importers of FDA-regulated products. You agent must meet some basic requirements, which we explain in this post about how to choose a US agent.
