 Matt Traynor, PhD
Matt Traynor, PhD
 Matt Traynor, PhD
Matt Traynor, PhD
1-Minute Summary
- Bemotrizinol is under FDA review, with the Agency issuing a proposal to add it as a permitted active ingredient in December 2025. Public comment is now open.
- Globally used in sunscreens, Bemotrizinol offers broad-spectrum protection, high photostability, and low skin absorption.
- Certified Laboratories offers a validated method for both Bemotrizinol and Bisoctrizole – one of the first in the U.S.
- Manufacturers should begin R&D now to prepare for potential regulatory approval and gain first-mover advantage.
U.S. Sunscreen Innovation May be About to Change
For decades, U.S. sunscreen innovation has lagged behind global markets. While Europe, Asia, and Australia have long used next-generation UV filters like Bemotrizinol and Bisoctrizole, U.S. formulators remain limited to an aging list of approved actives.
But that may soon change. In December 2025, the FDA announced a proposal to add bemotrizinol as a permitted active ingredient in over-the-counter (OTC) sunscreen products.
The FDA is now seeking public comments on the proposed order before issuing a final decision, but it’s clear that a new regulatory pathway is underway that could reshape the future of American sunscreen formulations. If you’re a manufacturer of SPF products, now is the time to pay attention and start preparing.
Bemotrizinol and Bisoctrizole: Where We Are Today
Let’s start with the basics.
Bemotrizinol (also known as Tinosorb S or PARSOL Shield) is an advanced, oil-soluble UV filter that absorbs both UVA and UVB rays. It’s been widely used in Europe and Asia for over two decades. The same goes for Bisoctrizole (Tinosorb M), a hybrid UV filter that works by absorbing, scattering, and reflecting UV radiation.
However, neither compound is currently approved in the U.S. as an active ingredient in over-the-counter (OTC) sunscreens.
That’s slowly changing, at least for Bemotrizinol.
In October 2024, DSM-Firmenich submitted an OTC Monograph Order Request (OMOR) to the U.S. FDA seeking approval of Bemotrizinol as a GRASE (Generally Recognized as Safe and Effective) sunscreen active. The FDA is now seeking public comments as of December 2025.
Let’s be clear: this is not guaranteed. The FDA may request additional data, which could delay approval. But the pathway is active, the application is complete, and public comments are being accepted.

Why Bemotrizinol Sunscreen Is a Game-Changer
There’s a reason Bemotrizinol sunscreen is the go-to choice in global formulations.
1. Broad-Spectrum Power
With dual absorption peaks around 310 nm (UVB) and 340 nm (UVA), Bemotrizinol offers robust, long-lasting coverage across the spectrum. This enables high SPF ratings with lower overall concentrations.
2. Photostability
Unlike avobenzone, which quickly degrades in sunlight, Bemotrizinol holds strong. Even better, it stabilizes less photostable filters, improving the durability of your entire formulation.
3. Skin-Friendly and Safe
It’s virtually water-insoluble and has a large molecular size, limiting dermal penetration. That translates to low systemic absorption and minimal irritation, making it a preferred choice for sensitive skin products.
4. Formulation Flexibility
Its oil-soluble nature works beautifully in emulsions and creams, pairing well with other organic filters or mineral actives like zinc oxide.
Bisoctrizole: A Strong Companion, With U.S. Limitations
Bisoctrizole, or Tinosorb M, brings its own advantages. It’s a particulate filter that’s both organic and inorganic in nature. Water-dispersible and photostable, it provides excellent coverage and often appears alongside Bemotrizinol in international products.
However, in the U.S., Bisoctrizole cannot be marketed as an SPF active. It may be used as a cosmetic UV absorber – say, in tinted moisturizers or primers – but any SPF claim must come from approved actives. So while it’s a helpful supporting filter, it can’t lead the charge like Bemotrizinol could.
Zeroing in on a Final Decision
If the FDA issues a final decision, it would make bemotrizinol the first new sunscreen active approved in the U.S. in over 20 years.
But here’s the catch: even if approval is granted, it will take time before these products hit store shelves. Manufacturers will need to update formulations, test for stability, validate claims, and comply with labeling and GMP requirements.
Waiting for a final decision to start this process means you’ll already be behind.
Now Is the Time to Prepare Your Bemotrizinol Sunscreen Formulations
We recommend manufacturers begin research and development now for a few reasons:
- Formulation development can take 6–12 months.
- Raw material sourcing must be vetted and secured in a volatile market.
- SPF efficacy, in-package stability, and preservative efficacy testing must be planned and executed.
- Label and regulatory reviews will be required after approval.
Forward-thinking brands that start now can be first to market once Bemotrizinol is cleared for use in U.S. sunscreens.
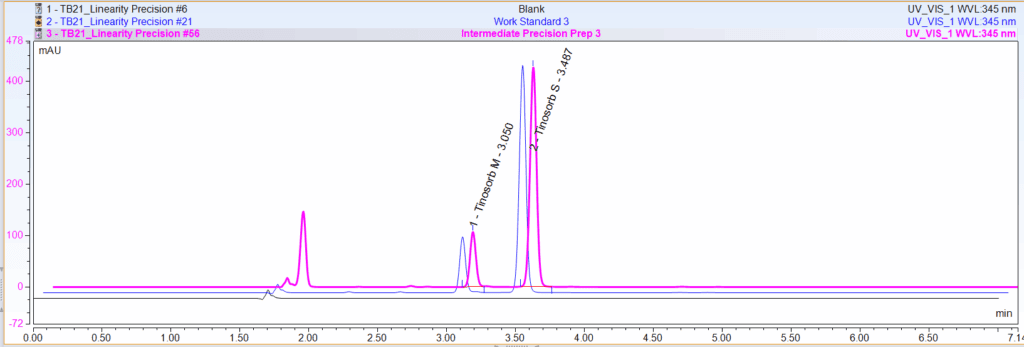
Certified Laboratories Has Validated Methods for Bemotrizinol and Bisoctrizole
Formulators often face a hurdle: testing. Fortunately, Certified Laboratories has a solution.
We are proud to be one of the first U.S. laboratories with a validated method for both Bemotrizinol and Bisoctrizole.
Our method offers…
- 7-minute run time per analysis, ensuring high throughput and fast turnaround.
- Compliance with ICH Q2(R2) guidance, helping ensure reliability and regulatory alignment.
- Dual-analyte capability, analyzing both Bemotrizinol and Bisoctrizole in a single run.
Whether you’re developing a Bemotrizinol sunscreen, a hybrid formula using Bisoctrizole, or a product for global distribution, our lab is ready to help.
Contact Us Today
There’s no need to wait for a green light to start building your next-generation SPF product. If you’re formulating with Bemotrizinol – or planning to – Certified Laboratories can test your samples now using validated, reliable methods.
