1-Minute Summary
- Cosmetics microbiology testing verifies that products are free of harmful microbes and safe for consumer use.
- Testing is performed at multiple stages – water, raw ingredients, in-process materials, and finished goods.
- USP <51>, <61>, and <62> are the primary methods used in ISO 17025-accredited labs.
- Microbiology testing supports compliance with MoCRA and retailer-specific requirements (e.g., Amazon ophthalmic and skin-lightening product policies).
How Cosmetics Microbiology Testing Fits into Safety Substantiation
At Certified Laboratories, we regularly hear from new cosmetics brands, contract manufacturers, and first-time formulators who ask…
- What testing do I need to do?
- What are the regulations?
- How do I make sure my product is safe to sell?
This article is for you. Whether you’re launching a startup or scaling your line, we’ll walk you through the fundamentals of cosmetics microbiology testing, including what it is, when it’s done, and why it matters.
Step-by-Step: Microbiology Testing of Cosmetics
Microbiology Testing Protects Consumers (and Your Brand)
Cosmetics are applied directly to the skin – often around the eyes, lips, or over damaged skin. Even a small amount of microbial contamination can lead to irritation, infection, or product spoilage. Consumers may never see the lab work that goes into your product, but they certainly feel it when something goes wrong.
That’s where microbiology testing comes in. It’s a key tool for verifying that your product is safe, free of objectionable organisms, and stable for use throughout its shelf life.
What Is Cosmetics Microbiology Testing?
Microbiology testing involves evaluating your product – or the materials used to make it – for microbial contamination. At Certified Laboratories, our ISO 17025-accredited cosmetic testing labs use validated compendial methods found in the United States Pharmacopeia (USP):
- USP <61> – Microbial Enumeration: Measures the total number of aerobic bacteria.
- USP <62> – Tests for Specified Microorganisms: Screens for harmful organisms like E. coli, S. aureus, Pseudomonas aeruginosa, and Candida albicans.
- USP <51> – Antimicrobial Effectiveness Test (Preservative Challenge Test): Evaluates how well your product resists microbial growth over time.
These methods are considered the gold standard. In our lab, they’re performed under strict Standard Operating Procedures (SOPs) using calibrated incubators, validated media, and expert-trained microbiologists.
A Look Inside: How We Perform Micro Testing at Certified Laboratories
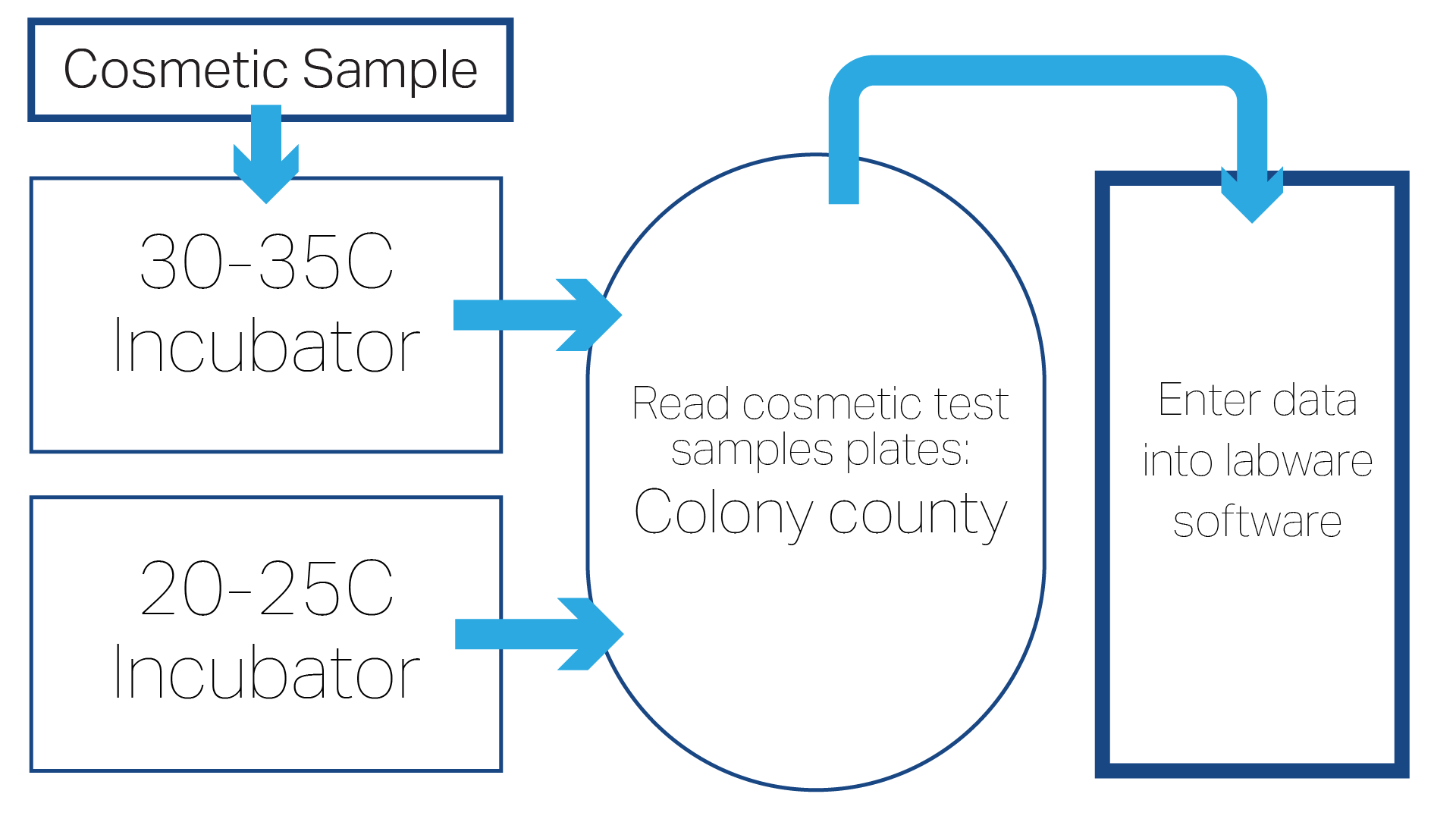
Our microbiologists follow these steps when testing cosmetics samples in our laboratories:
Key Micro Testing Steps
- Receive samples in the lab using aseptic techniques to ensure sample integrity.
- Neutralize product in nutrient broth.
- Transfer into petri dishes and add agars.
- Incubate: 30° to 35°C (3 days).
- Incubate: 20° to 25°C (5 to 7 days).
- Perform enrichment step.
- Read plates: using colony counter with light source and magnifying glass, look for bacteria growth on tryptic soy agar (TSA) plates. Look for yeast/mold growth on potato dextrose agar (PDA) plates.
- Following Standard Operating Procedure (SOP), enumerate any microbial growth and characterize any potentially pathogenic microorganisms (e.g. Staphylococcus aureus).
- Enter data into the LIMS/Labware system.
- Review, release Certificate of Analysis (COA) (summary of data) to customer.
When Is Cosmetics Microbiology Testing Performed?
To get a clear picture of quality and safety, micro testing is performed at multiple stages:
- Water: Whether used in the formulation or manufacturing process, it is one of the most common sources of contamination in cosmetics.
- Raw Ingredients: Especially botanical or natural materials.
- Bulk/In-Process: During mixing or compounding phases.
- Finished Product: Before it’s released to market.
This stepped approach helps manufacturers detect and prevent issues early – before a contaminated batch ever reaches the warehouse.
Regulatory Landscape: Why Testing Matters Now More Than Ever
Cosmetic manufacturers are legally responsible for ensuring that their products are safe for consumer use. That’s not new, but the expectations have changed under the Modernization of Cosmetics Regulation Act (MoCRA).
Manufacturers must now have adequate safety substantiation on file before selling a cosmetic product in the U.S. This includes scientific evidence that your product, when used as intended, is not harmful to consumers.
While MoCRA doesn’t spell out every test required, microbiological testing is a critical part of demonstrating safety, especially if…
- Your product contains water or natural ingredients.
- It’s used near the eyes or mouth.
- You’re using a new preservative system.
- Your product is intended for sensitive skin or high-risk populations.
What About Amazon Requirements?
Amazon has its own microbiology-testing requirements for ophthalmic and skin-lightening products sold on its platform, which we explain here. These products are considered over-the-counter (OTC) drugs, which means the bar is higher for safety.
In short, while U.S. regulations don’t mandate specific tests, you must substantiate product safety, and micro testing – along with ingredient reviews, toxicological assessments, analytical chemistry, in-package stability testing, and other tools – help your company meet this requirement.
It All Starts in the Lab
At Certified Laboratories, our microbiologists are here to help ensure your product is safe, effective, and compliant.
Whether you’re launching your first product or refining your testing strategy, we’re here to support you – please contact us if you need cosmetics microbiology testing or have questions.

