Reviewed and Approved by Matt Traynor, PhD, Vice President, Innovation and Validation, Certified Laboratories
1-Minute Summary
- Ido-BR1 has shown promise in clinical trials as an anti-inflammatory for joint health, making it a popular ingredient in joint-health supplements.
- U.S. FDA regulations in 21 CFR 111 require identity testing for each ingredient in supplement formulations, including Ido-BR1.
- HPLC and LC-MS/MS are used, depending on the sample type.
- Certified Laboratories offers a validated method with 14-day TAT and LOD/LOQ of 50 ppb/100 ppb (0.05 mg/kg / 0.1mg/kg).
Certified Laboratories’ Introduces Validated Ido-BR1 Method for Supplements
The joint health category tallied about $2.25B in 2024, and demographics point to continued expansion as preserving mobility is a top concern of supplement users.
With that in mind, Certified Laboratories now offers a validated method for Ido-BR1 – a compound in certain cucumber extracts used in many joint-health supplements due to its anti-inflammatory properties.
Validated Ido-BR1 Method
- Sample Types: Gummies, Tablets, Capsules
- TAT: 14 Business Days
- LOD/LOQ: 50 ppb / 100 ppb (0.05 mg/kg / 0.1mg/kg)
What Is Ido-BR1 and Does it Work?
Ido-BR1 is a small, sugar-like molecule (an iminosugar amino-acid analog) naturally present in certain cucumber extracts used in joint formulas.
Just as important, the science around Ido-BR1 and its standardized extract offers promise for joint health. In lab and ex vivo models, Ido-BR1 reduced inflammatory signaling and showed selective enzyme interactions consistent with anti-inflammatory activity.
And in humans? Multiple randomized studies of Q-Actin®, a cucumber extract standardized to >1% Ido-BR1, report meaningful joint outcomes at very low daily doses:
- A 6-month, multicenter RCT (n=122) using 10 mg twice daily showed significantly greater improvements in WOMAC pain/stiffness/function than glucosamine–chondroitin, with no reported adverse effects.
- A 180-day, placebo-controlled trial tested 20 mg and 100 mg/day and found dose-dependent improvements in pain-related parameters linked to moderate knee osteoarthritis.
These findings help justify why Ido-BR1 is a popular choice when formulating joint-health supplements.
Why Conduct Ido-BR1 Testing on Your Supplements?
There are two main reasons to conduct ido-BR1 testing on your samples: compliance and confidence.
- 21 CFR 111. U.S. FDA regulations mandate identity testing for each dietary ingredient. Plus, you must establish specifications for identity, purity, strength, and composition, which requires testing to ensure those specs are met.
- Label support & efficacy alignment. Verification helps ensure that per-unit delivery aligns with your intended dose and label claims.
- Stability assurance. Moisture, heat, and sugars can challenge actives in gummies. Trending Ido-BR1 during stability testing shows that your product stays within your criterion over its shelf life.
- Risk mitigation. Early detection of adulterated or out-of-spec raw materials prevents sub-potent or non-conforming release.
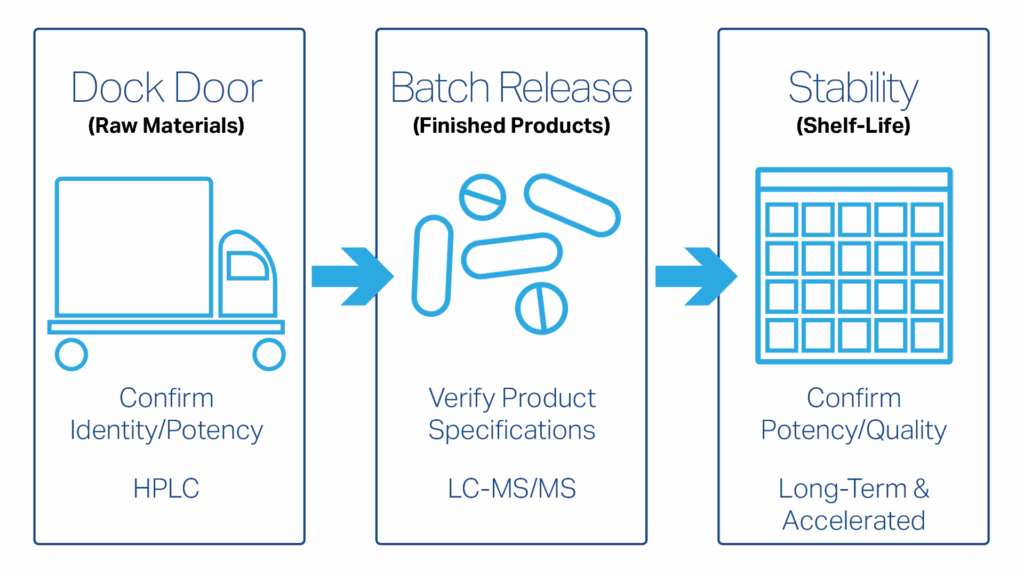
When Should I Conduct Ido-BR1 Testing?
Start at the dock door. When a raw material arrives, confirm its identity and that its Ido-BR1 potency matches what the supplier claims. This identity/potency check tells you what you’re putting into the blend.
For batch release, test finished units to ensure milligrams of Ido-BR1 per capsule, tablet, or gummy meet your specifications.
Finally, carry the same thread into stability. Run long-term and accelerated stability studies and trend Ido-BR1 against your specifications across storage conditions. The result is a clear picture of how the compound holds up over the product’s shelf life.
When to Test Ido-BR1 in Your Process
A Look at Certified Laboratories’ Ido-BR1 Validated Method
Our ISO 17025-accredited supplement testing labs take different approaches for Ido-BR1 testing, depending on the sample type. HPLC gives you speed and reliability on ingredients; LC–MS/MS gives you confidence in complex finished goods. Using the right tool at each step keeps decisions simple and sound.
Raw Materials Testing
For raw materials like extract powders, we run a straightforward liquid-chromatography test (HPLC) to confirm identity and the % Ido-BR1 in each incoming batch. It’s quick, consistent, and ideal for identity and potency checks.
Finished Products Testing
For finished products such as gummies, capsules, and tablets, we use LC–MS/MS. This instrument offers increased sensitivity, which is vital for complex matrices that often include sugars, gels, and other “noise”. We report results as mg/kg or mg per unit.
Common Testing Pitfalls to Avoid
Everyone needs accurate results as soon as possible. To help avoid common pitfalls that slow down the process, keep these points in mind:
- For lab intake, please send ≥10 g of sample so we can run replicates, matrix checks, and re-injections without delays.
- For gummies, sharing basics of the gel/sugar system speeds troubleshooting if interferences pop up.
- If testing raw materials, send us the supplier’s Certificate of Analysis, which helps ensure our lab has all required information and reduces callbacks.
Start Your Ido-BR1 Testing
If you need Ido-BR1 lab testing for your raw materials or finished products, contact our team today. Our ISO 17025-accredited labs are ready to process your samples today!
